Electrolysis of Water; Electrolytic Hydrogen Generators
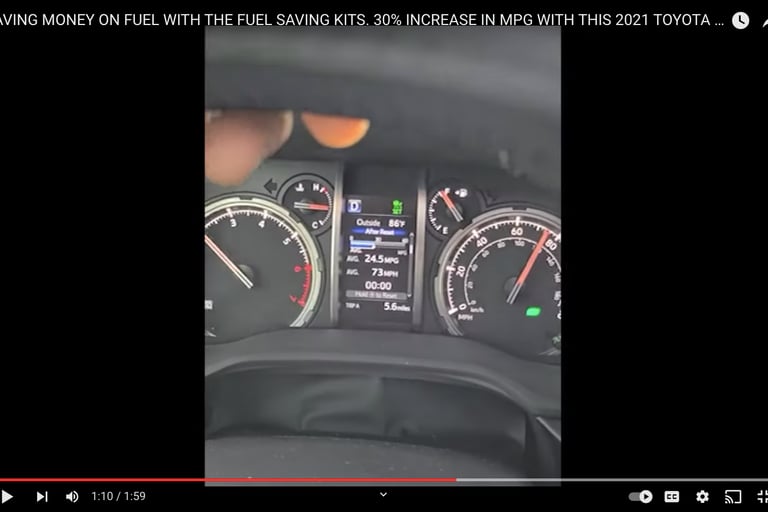
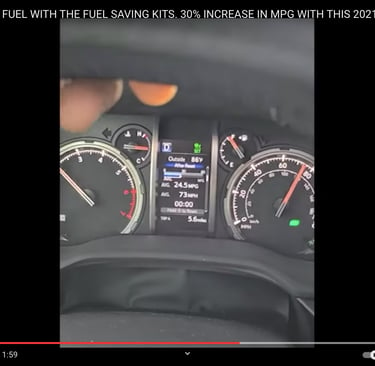
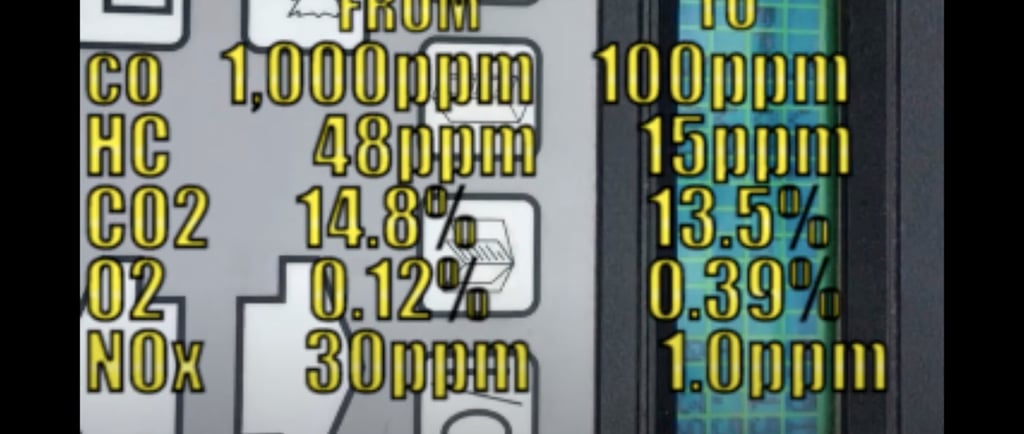
Our HHO injection kit uses electrolysis to efficiently produce hydrogen with our TITANIUM core/cell HHO generator and fuel injects using electrolysis of water.
TRANSPORTGREEN VEHICLECARSBUSINESSUSED CARSBCLOGISTICSFUEL ECONOMYVEHICLESDIESELAUTOMOBILE HYDROGENVANCOUVERGAS VEHICLESVACATIONHYDROGENENVIRONMENTHYDROGEN FOR CARSFINANCETRAVELTRUCKSEMISSIONSSAVE ON GAS AUTOSHHO OTTAWAZERO EMISSIONSHYBRID CARSMONTREALNET ZEROQUEBECFUELTORONTO
4/25/20243 min read

Electrolysis of water is a fundamental chemical process used to decompose water (H2O) into its constituent elements, hydrogen (H2) and oxygen (O2), through the application of an electric current. This process occurs in an electrolytic cell, a setup that includes two electrodes (an anode and a cathode) submerged in water, often with an electrolyte added to increase conductivity. When a direct current is passed through the water, hydrogen ions (protons) in the water are attracted to the cathode, where each gains an electron to become hydrogen gas. Simultaneously, at the anode, water molecules lose electrons to form oxygen gas and positively charged hydrogen ions. The overall chemical reaction involves the splitting of two water molecules to form two molecules of hydrogen gas and one molecule of oxygen gas, summarized by the equation 2H2O(l) → 2H2(g) + O2(g). Electrolysis of water is not only fundamental in the field of chemistry for the production of these gases but also increasingly significant in energy applications, particularly in the production of clean hydrogen fuel for energy storage and fuel cells, contributing to advances in sustainable energy solutions.
Electrolysis of Water


Hydrogen Generator
A hydrogen generator is a device that produces hydrogen gas, primarily through the process of electrolysis of water. This involves using an electric current to split water molecules into their basic components—hydrogen and oxygen.
HHO Generator
The hydrogen produced can be used for various applications, including as a fuel in hydrogen fuel cells, where it combines with oxygen to produce electricity, heat, and water, offering a clean alternative to fossil fuels. Hydrogen generators are found in a range of sizes, from small, portable units for laboratory use to large-scale industrial systems designed for energy production and storage.
A Hydrogen Eletrolyzer
They provide a sustainable solution by potentially using renewable energy sources to power the electrolysis process, thereby producing green hydrogen with minimal environmental impact. As demand for sustainable energy solutions grows, hydrogen generators are increasingly significant in sectors like transportation, where they help power vehicles, and in industrial processes where clean energy is crucial.


Electrolyzer
What is Electrolysis? Green Hydrogen...
An electrolyzer is a device used to generate hydrogen gas through the electrolysis of water. This process splits water (H2O) into its constituent hydrogen (H2) and oxygen (O2) gases by passing an electric current through it. Electrolyzers consist of an electrolyte, electrodes, and an external power source. The electrolyte can vary, often being a solution like potassium hydroxide or a solid polymer electrolyte, while the electrodes are typically made from conductive materials like platinum or iridium. Do HHO generators really work? Ours have a TITANIUM fuel cell. They work excellent.
How to make Hydrogen from Water
The role of the electrolyzer in the clean energy landscape is becoming increasingly important as the world shifts towards sustainable energy solutions. By using electricity derived from renewable sources, such as solar or wind, electrolyzers can produce "green hydrogen," which is a key element in reducing carbon footprints and achieving energy independence. This technology is vital for industries like transportation, manufacturing, and utilities, where it can serve as a clean, efficient fuel or a storage medium for excess energy generated from renewable sources.

Hydrogen Electrolysis
Hydrogen electrolysis is a process that splits water into hydrogen and oxygen using an electric current, a technique essential for producing pure hydrogen gas, particularly for fuel applications. In an electrolyzer, water, often enhanced with an electrolyte to improve conductivity, is subjected to an electric current via electrodes. This current dissociates water molecules, releasing hydrogen at the cathode and oxygen at the anode.
Electrolytic Hydrogen Generator
The potential of hydrogen electrolysis lies in its ability to harness electricity from renewable sources like wind or solar, thus producing "green hydrogen." As the world seeks sustainable energy alternatives, hydrogen produced via electrolysis offers a promising solution for energy storage, clean fuel production, and reducing greenhouse gas emissions, supporting a shift towards a more sustainable and energy-efficient future.